Mechanisms of RNA regulation in male germ cells
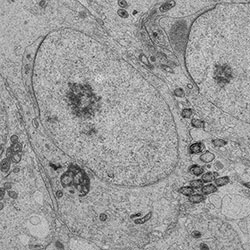
Protection of genetic and epigenetic integrity in the germline is crucially important to guarantee perpetuation of the species, production of healthy offspring, and evolution. Our research focuses on RNA regulatory mechanisms that govern complex gene expression changes during spermatogenesis. These mechanisms are essential for the maintenance of male fertility. Importantly, they may also contribute to the health of future generations by determining sperm RNA content that can mediate epigenetic inheritance of acquired conditions, such as metabolic disorders. One powerful mechanism to control transcriptome in the male germline is provided by germ cell-specific cytoplasmic ribonucleoprotein granules (germ granules) that appear at the time of active genome transcription. The most prominent granules are the intermitochondrial cement (IMC) between mitochondrial clusters in spermatocytes, and the chromatoid body (CB) in haploid round spermatids. We have shown that two important RNA regulatory pathways, PIWI-interacting RNA (piRNA) and nonsense-mediated RNA decay (NMD) pathways, accumulate in the CB together with a wide range of RNAs. By using mouse as a model, our research focuses on characterizing the functional role of germ granules in the posttrancriptional control of gene expression during spermatogenesis.
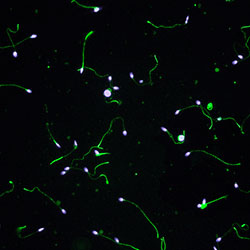
Sperm epigenome-mediated non-genetic inheritance
Recent research using animal models has shown that many acquired traits and conditions in fathers can be transmitted to the next generation(s) through the process of epigenetic inheritance. The health of subsequent generations may therefore depend on the health status of the future father at the time of conception, and it is likely that epigenetic inheritance contributes to the increased prevalence of non-communicable diseases, such as diabetes and other metabolic disorders in human populations. Obesity in adolescence has tripled in 40 years, and half of young men are overweight/obese in Finland today. Therefore, it is evident that understanding the contribution of epigenetic inheritance in the etiology of metabolic disorders has a major public health relevance.Paternal epigenetic inheritance is mediated via environmentally-induced changes in the sperm epigenome that are passed to subsequent generations in fertilization. Strong evidence points at an important role for sperm non-coding RNAs as intergenerational carriers of epigenetic information. It is still unknown how information about environmentally-induced phenotypic changes are converted into changes in sperm epigenome. Furthermore, recent studies have shown that sperm epigenome appears to be dynamic, but we still need more detailed information about the stability of environmentally-induced changes, i.e. whether they can be reversed, and would this improve offspring health. The mechanisms of transgenerational epigenetic inheritance across several generations are also yet to be characterized. Our research actively explores answers to these key open questions. In addition to using mouse as a model, we are actively involved in human cohort studies to address the importance of epigenetic inheritance in human health and disease in collaboration with the Young Finns Study (YFS) led by Prof. Olli Raitakari, and the FinnBrain Study led by Prof. Hasse Karlsson.
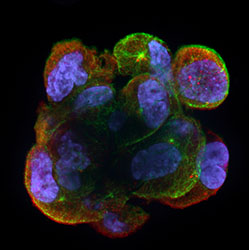
Germline-specific RNA regulatory mechanisms in cancer progression
Cancer cells often use their reservoir of normally silent genes to activate gene expression programs that support cancer progression. Many of the ectopically expressed cancer antigens are in fact genes that besides tumours are known to be specifically expressed in the male germline, and they are therefore collectively referred to as cancer/germline antigens. Cancer/germline gene expression levels are often correlated with tumour progression and clinical outcomes in various types of somatic malignancies. In order to thrive, form a tumour and metastasize, cancer cells need to adjust cell metabolism, migrate out of the original tissue, nest and survive in new microenvironments. To overcome these challenges they need to acquire many new capabilities. Therefore, it is a tempting hypothesis that ectopically activated cancer/germline genes are essential to satisfy the new requirements for cancer cells. Importantly, cancer/germline genes provide potential cancer therapy targets because of their restricted expression in the germline, which greatly reduces the risk for systemic side effects upon targeting. In our research, we are elucidating the importance of germ cell-specific RNA regulatory mechanisms in tumorigenesis and evaluate their potential as a cancer therapy targets.